Visible Light-Promoted CO2 Fixation with Imines to Synthesize Diaryl α-Amino Acids
Xinyuan Fan,* Xu Gong,# Mengyue Ma,# Rui Wang, and Patrick J. Walsh*
a Institute of Advanced Synthesis, School of Chemistry and Molecular Engineering, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing, 211816, P. R. China
b Roy and Diana Vagelos Laboratories, Department of Chemistry, University of Pennsylvania, 231 South 34th Street, Philadelphia, Pennsylvania 19104-6323, United States.
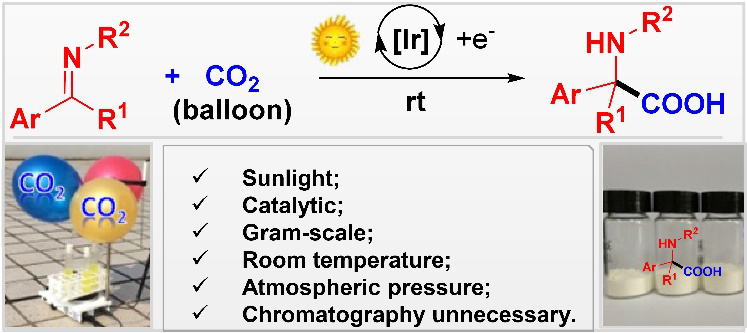
Abstract:Light-mediated transformations with CO2 have recently attracted great attention, with the focus on CO2 incorporation into C–C double and triple bonds, organohalides and amines. Herein is demonstrated visible light -mediated umpolung imine reactivity capable of engaging CO2 to afford α-amino acid derivatives. By employing benzophenone ketimine derivatives, CO2 fixation by hydrocarboxylation of C=N double bonds is achieved. Good to excellent yields of a broad range of α,α–diaryl α-amino acid derivatives are obtained under mild conditions (rt, atmospheric pressure of CO2, visible light). A procedure that avoids tedious chromatographic purification and uses sustainable sunlight is developed to highlight the simplicity of this method.
Nature Communications2018, DOI: 10.1038/s41467-018-07351-2. (2017年影响因子: 12.353).
论文链接:https://doi.org/10.1038/s41467-018-07351-2
